OUR Pipeline
Our inhaled-AAT platform is built on a modular approach that allows us to efficiently develop products targeting high-need inflammatory and infectious lung diseases. Our products are covered by a portfolio of own IP.


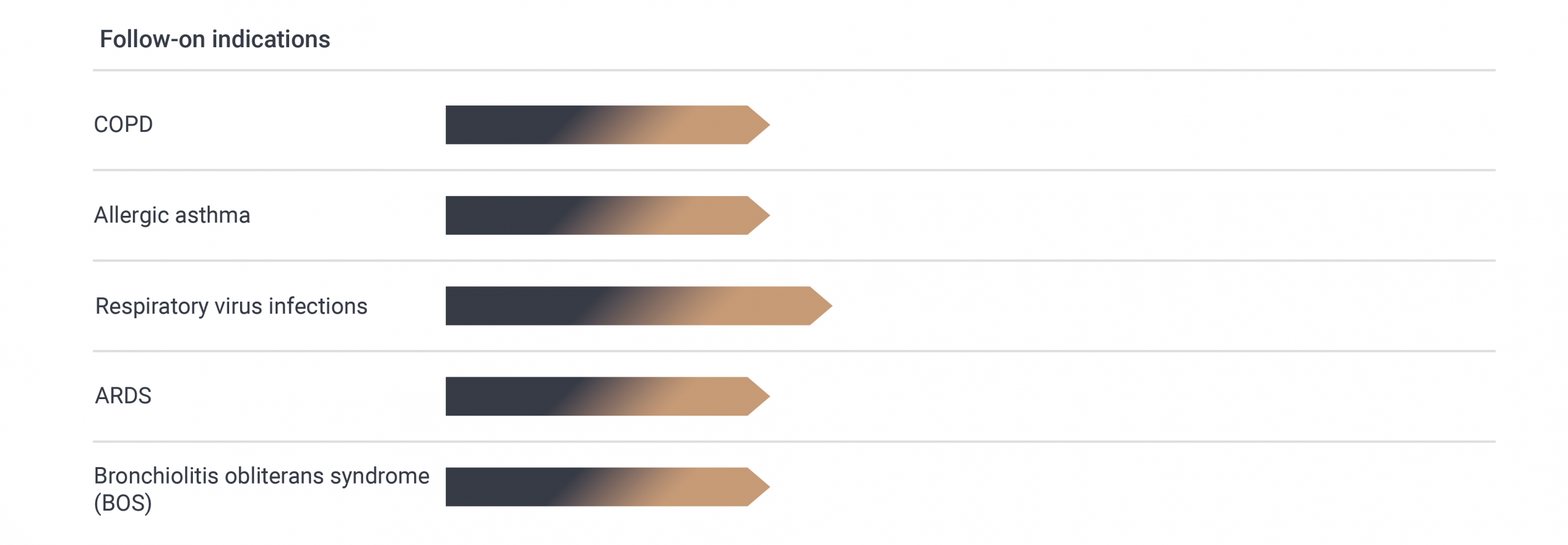
First Clinical Study Planned in 2026
A Phase 1b clinical study design has already been agreed upon with the US FDA and the German Paul-Ehrlich-Institut (PEI), with trials in NCFB patients planned to start in 2026.
De-risked Approach
Extensive preclinical proof-of-principle data demonstrate that ATL-105 effectively targets the key pathological processes of lung inflammation. As a recombinant version of AAT, a naturally occurring human protein, ATL-105 offers an excellent safety profile and minimal risk of immunogenicity, providing a highly predictable and low-risk path to clinical development.
Industrial scaling
Through our strategic partnership with Northway Biotech, we have established a robust manufacturing process, ensuring scalability and significantly higher purity than traditional plasma-derived AAT production.
Handheld inhalation
ATL-105 will be delivered via our proprietary handheld nebulizer, developed in collaboration with Beurer, optimized for deep lung deposition and ease of home use.